Extraction Of Caffeine From Tea Lab Report Answers
The melting point of caffeine is 228 6.
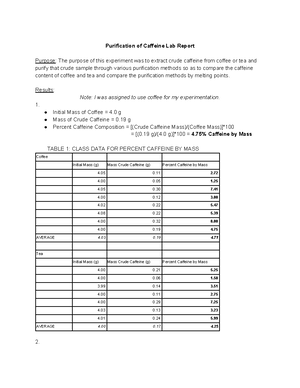
Extraction of caffeine from tea lab report answers. The amount of caffeine in a tea bag is 0 0979 g. After washing the anhydrous calcium chloride pellets with more dcm the solvent was evaporated leaving. View lab report lab report 5 from organic ch 112b at university of california berkeley. By using separatory funnel the caffeine was separated from tea and coffee dissolved in.
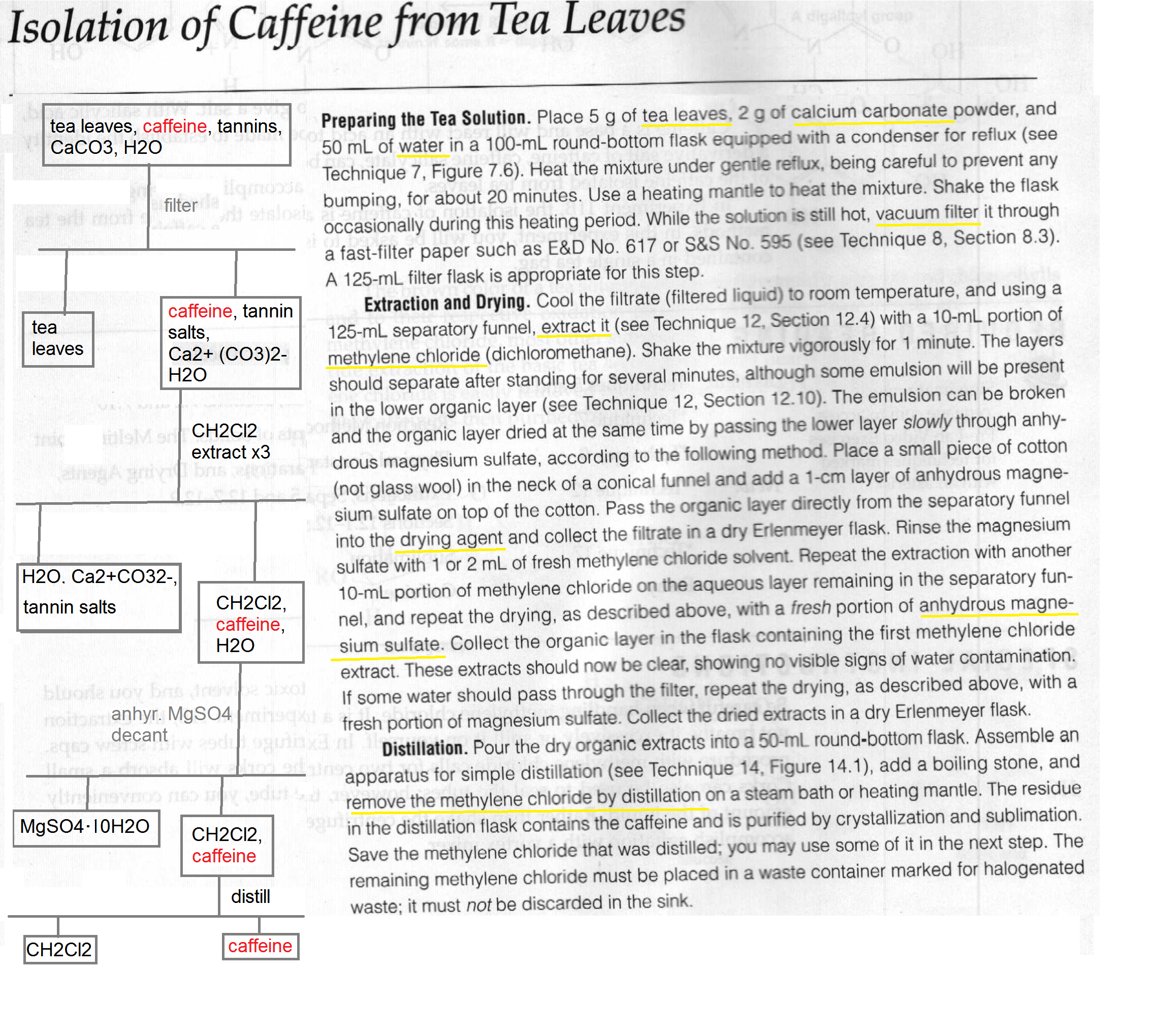
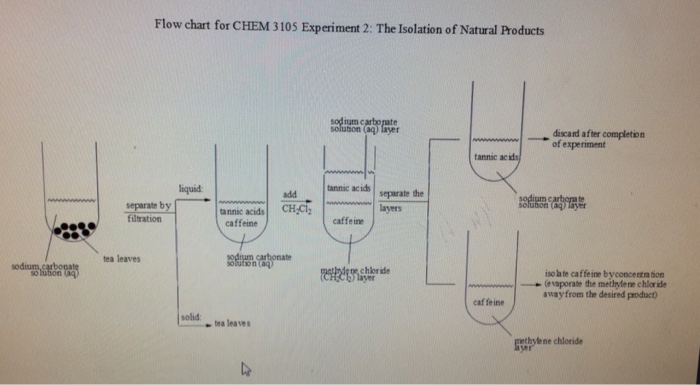
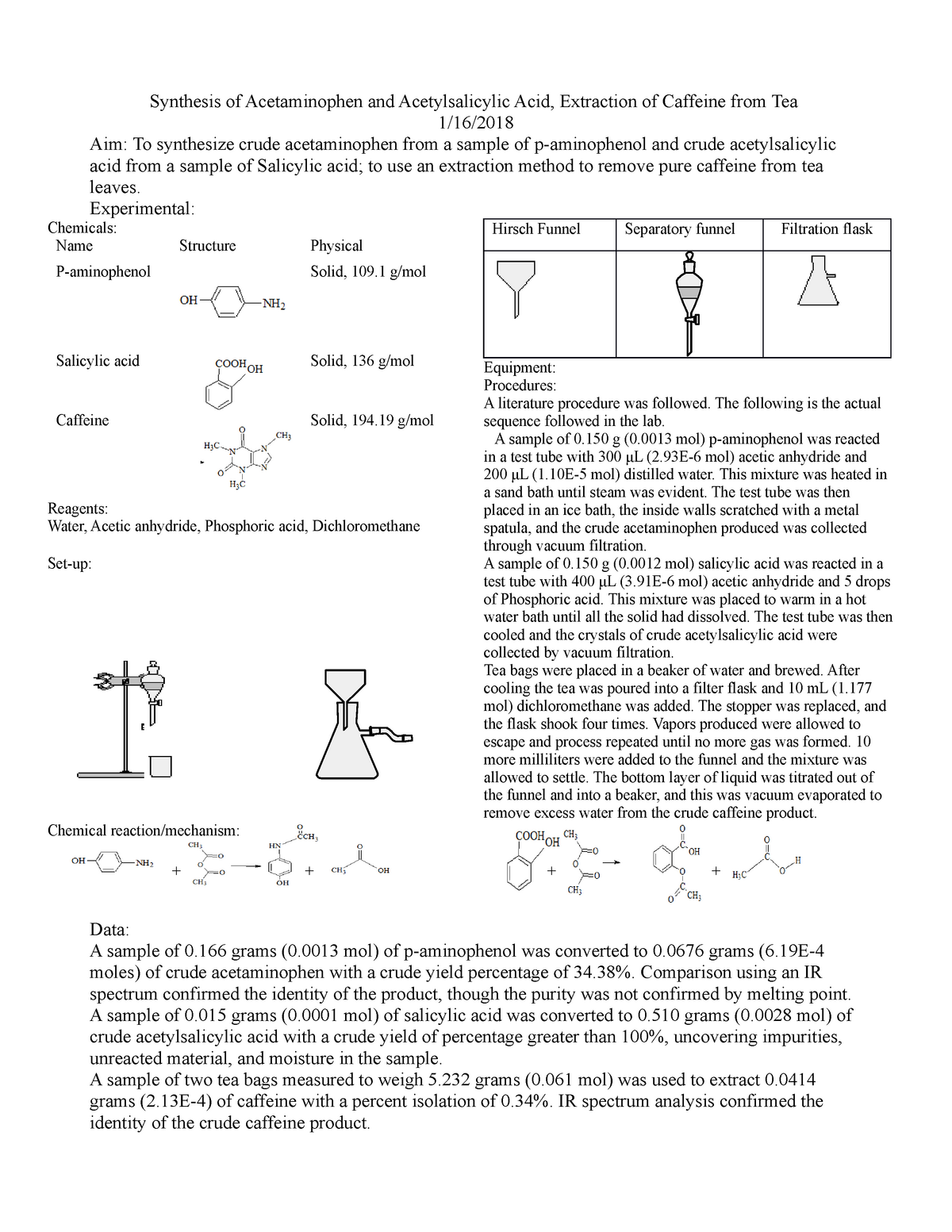
The mass of this solid would reflect the actual yield of caffeine in the tea. Caffeine lab 1 isolation of caffeine from tea in this experiment caffeine will be extracted from tea leaves where it is about 5 present using hot water. The purpose of this experiment was extraction of caffeine. The solvents used in the experiment were an aqueous sodium carbonate and dichloromethane dcm.
Anhydrous calcium chloride pellets were used to dry the solution and emulsion layer and the dcm was then decanted. Product found in coffee and tea. Organic chemistry i che 223 uploaded by. This gave calculated values of 59 1 recovery and 40 9 error.
Caffeine can be extracted easily from tea bags. It is to be noted that its scale up can be done according to. Caffeine was extracted from tea by the use of solid liquid and liquid liquid extractions. The caffeine was evaporated and appeared in yellowish form.
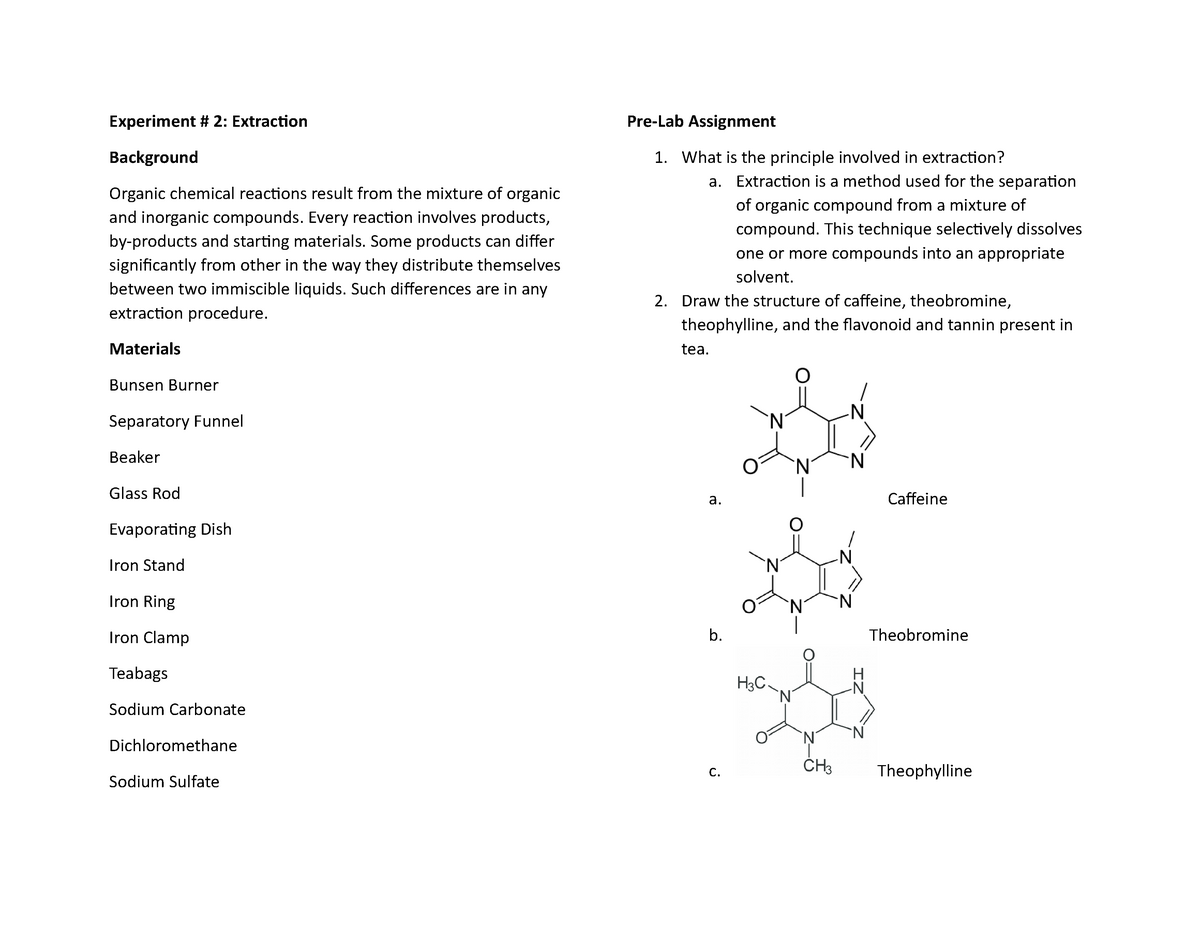
Extraction is a technique in which a solvent is used to remove and isolate a compound of interest from a liquid substance. Standard tea bags contain 2 00 0 05 g of tea leaves along with approximately 55 mg of caffeine. Che 223 lab report ysatis m fenner title extraction isolation of caffeine from coffee preformed october 1st 2018 submitted october 15th 2018 abstract caffeine. The purpose of this experiment was to perform a liquid liquid extraction method to extract the caffeine from the tea bags that were provided and then recrystallize the caffeine.
The product that was collected after extraction still had many impurities. Isolation of caffeine section 12 02 nattanit trakullapphan nam narissara pracharktam nik thaksaporn sirichanyaphong may abstract. Please sign in or register to. Conclusion in this experiment the caffeine was extracted from the tea bag.
This is essentially the same procedure used to decaffeinate drinks such as coffee and tea. Using the proper extraction methods the caffeine within a tea bag could potentially be isolated to yield a pure solid. The percentage yield of caffeine is 89 98. A pure product of 065 g caffeine was obtained.
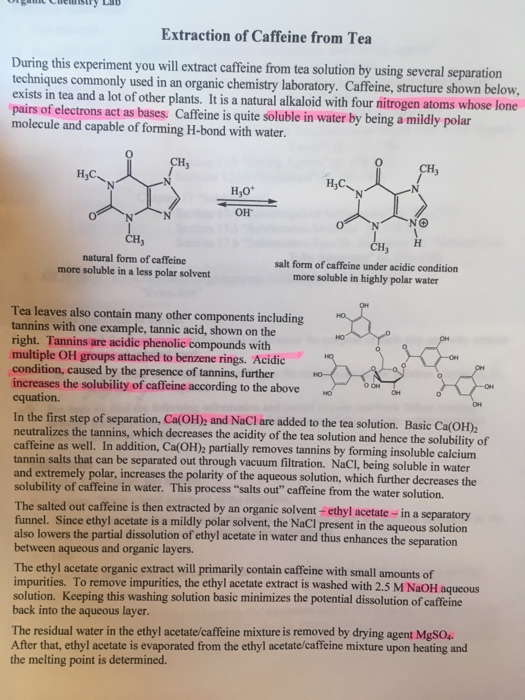
10 4 13 isolation of caffeine from tea objective. The procedure was simply steeping the tea with very hot water for a few minutes which aided in extracting most of the caffeine. Efficient extraction of caffeine from coffee relies heavily on the properties of caffeine other components present in coffee. Caffeine can be extracted from tea by its ability to be better dissolved in dichloromethane than water.