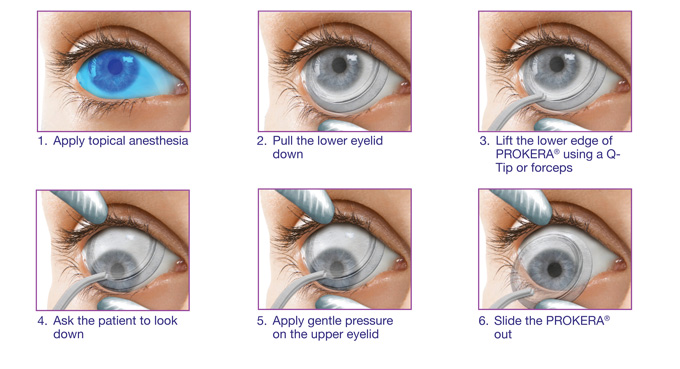
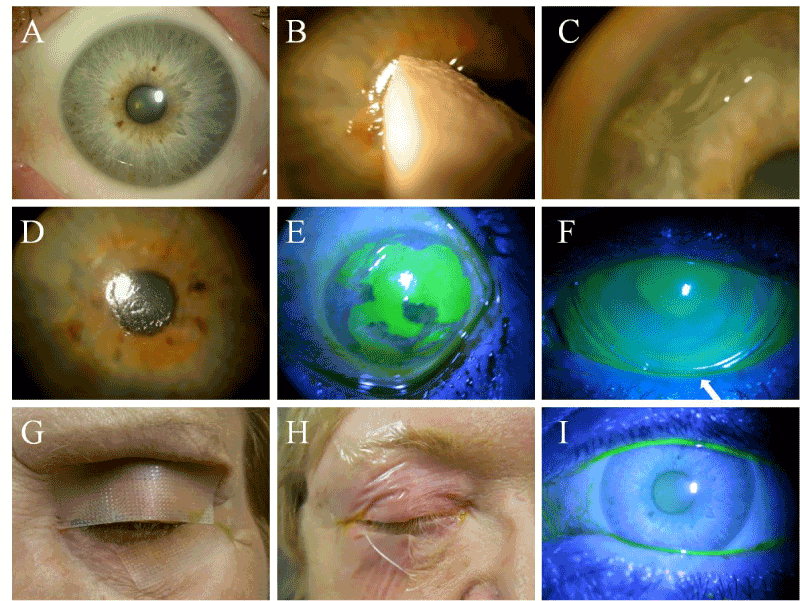
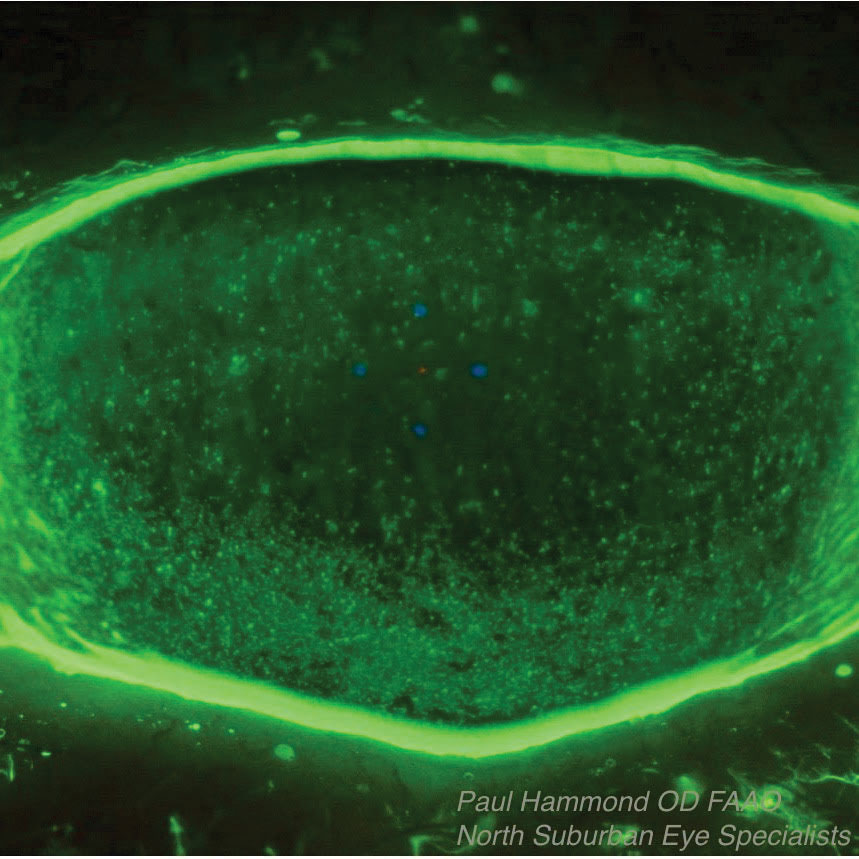
Prokera Ring Placement

Placement of amniotic membrane on the ocular surface.
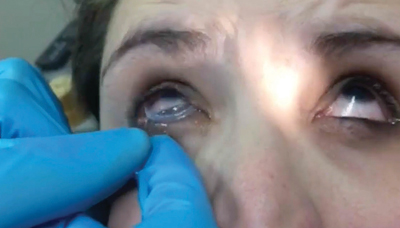
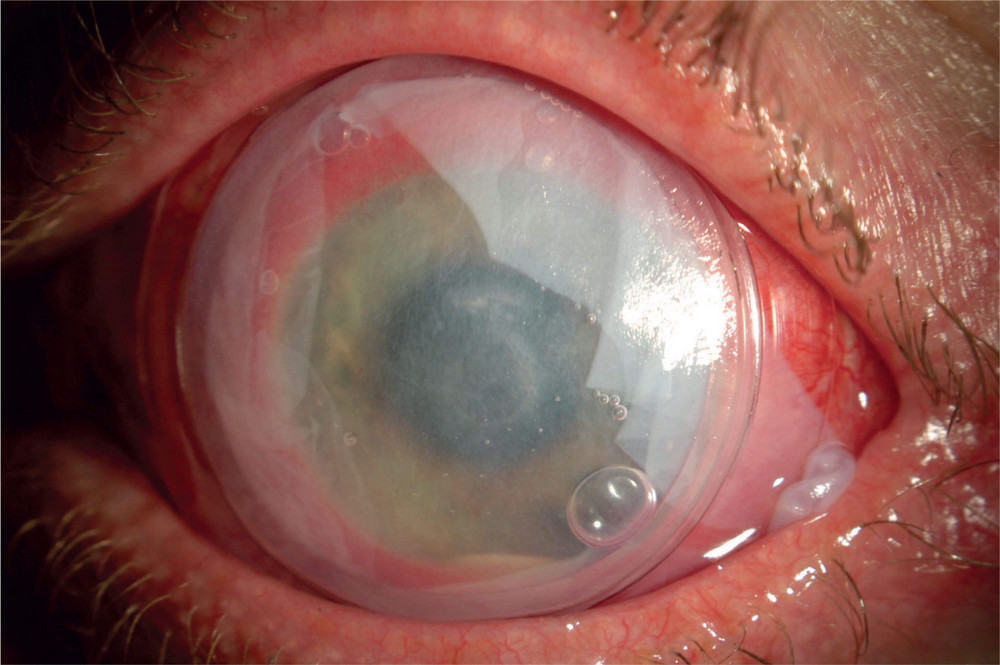
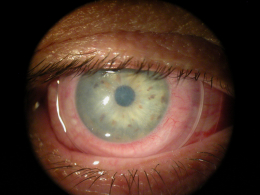
Prokera ring placement. Prokera is a medical contact lens device that can be fit into a diseased cornea with chronic inflammation when typical medications or medical treatment fails. It is inserted between the eyeball and the eyelid and is self retained on the eye. The dehydrated form ambiodisk iop ophthalmics costa mesa california is a sutureless overlay am disk. Prokera for patients prokera is a combination medical device used by eye doctors around the world for anti inflammation anti scarring and promoting healing of damaged eye surfaces.
Learn to code cornea. Prokera is a therapeutic device used by eye doctors around the world to protect repair and heal damaged eye surfaces. This dual action device can be easily inserted by eye care providers in office. The cryopreserved version prokera bio tissue doral florida includes a solid plastic ring that encompasses the cornea and sits on the conjunctiva.
It contains the only fda cleared cryopreserved amniotic membrane which supports the corneal healing process without harmful side effects. 65779 placement of amniotic membrane on ocular surface. The special contact lens must pass many quality control standards before being used by your doctor. 65778 placement of amniotic membrane on the ocular surface.
Prokera is available in 3 versions slim traditional and plus and the dehydrated form also includes. Also known as amt. The american medical association created cpt code 65778 currently defined as. Without sutures with a 10 day global period because it recognized the importance of delivering the wound healing properties of cryopreserved amniotic membrane to the ocular surface without the use of sutures.
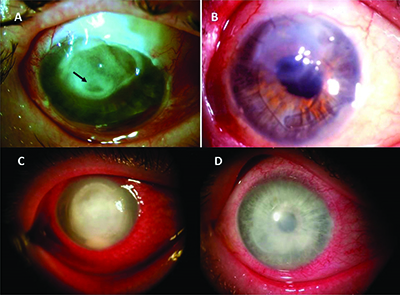
Prokera has two code choices. The mechanism is similar to a contact lens with a ring at the periphery that is filled with a clear natural amniotic membrane material. Prokera biologic corneal bandage devices are used by eye doctors around the world to heal and treat ocular conditions such as keratitis moderate to severe dry eye disease recurrent corneal erosions filamentary keratitis persistent epithelial defects neurotrophic corneas herpetic ulcers and many other ocular surface diseases such as chemical burns and stevens johnson syndrome. It is safe and effective and the tissue is regulated by the fda.
A according to the manufacturer the prokera family of products are corneal epithelial inserts consisting of an ophthalmic conformer that incorporates one or two layers of cryopreserved amniotic membrane. Prokera is the only fda cleared therapeutic device that can both reduce inflammation and promote healing. Aaoe board chair talks annual meeting. Amniograft bio tissue does not.