Purified Water System In Pharmaceutical Industry Pdf
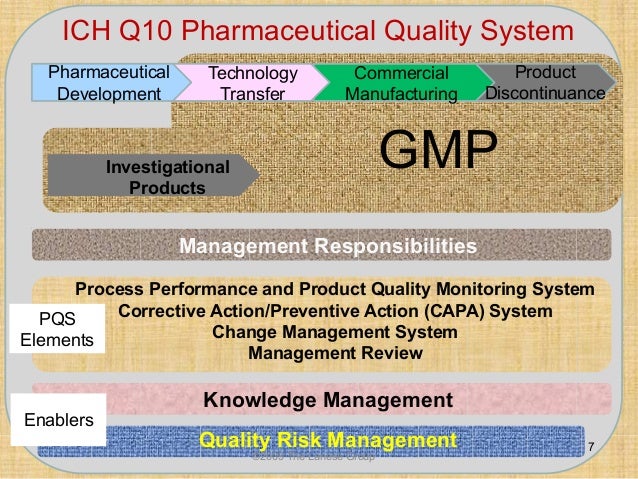
6 2 materials that come into contact with systems for water for pharmaceutical use 78 6 3 system sanitization and bioburden control 80 6 4 storage vessel requirements 80 6 5 requirements for water distribution pipework 81 7.
Purified water system in pharmaceutical industry pdf. Eur provides quality standards for grades of water for 36 pharmaceutical use including water for injections wfi purified water and water for preparation of 37 extracts. Water purifcatn in te pharmaceutical industry ad randc 007 e rev. Ppt pdf pharmaceutical water system validation identification of microorganisms identification of microorganisms pharmaceutical warer system ppt as stated above alert and action levels for a given process control attribute are used to help maintain system control and avoid exceeding the pass fail specification for that attribute. 33 pharmaceutical industry devotes considerable resource to the development and maintenance of water 34 purification systems.
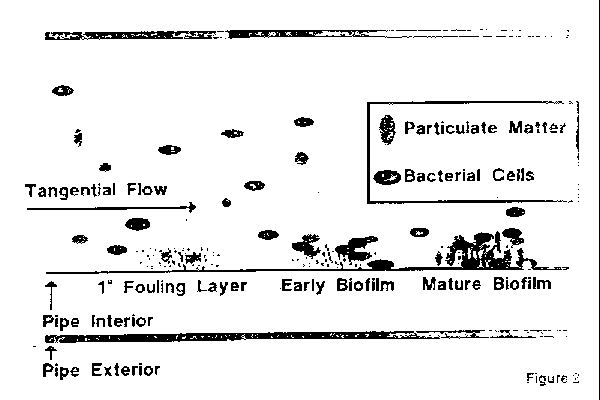
Water purification systems in pharmaceutical manufacturing. Validation and control of deionized water systems daniel l. High purity water systems 7 93 ispe baseline. Operational considerations 83 7 1 start up and commissioning of water systems 83 7 2 qualification 83 7 3 continuous.
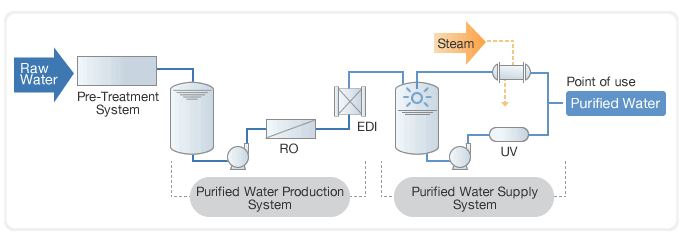
Water for injection wfi usp jp permits distillation or a purification process that is equivalent or superior to distillation in the removal of chemicals or microorganisms. There is no technical data in the industry to support the belief that rouge. The overall objective is to remove the impurities in the feedwater while minimising additional contamination from the components of the purification system and from bacterial growth. Water system these are the articles on purified water system and water for injection wfi system to produce purified water and water for injection used in pharmaceutical manufacturing those are helpful to new as well as experienced pharmaceutical professionals.
This page is updated regularly therefore don t forget to visit it again. Pharmaceutical water systems first edition 1997 theodore h. Permit production by. Fda inspection technical guide number 36 reverse.
Purified water water for injection and water for hemodialysis. General chapter 1231 of the usp defines three grades of monographed water for pharmaceutical purposes. Purified water parameter unit usp ph. Michels bureau of drugs aug.
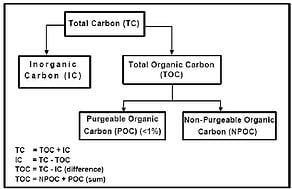
Bulk toc ppb c 500 500 conductivity 6s cm 20 c 4 3. 35 the european pharmacopoeia ph. Purified water pw normally the. Fda letter to the pharmaceutical industry re.
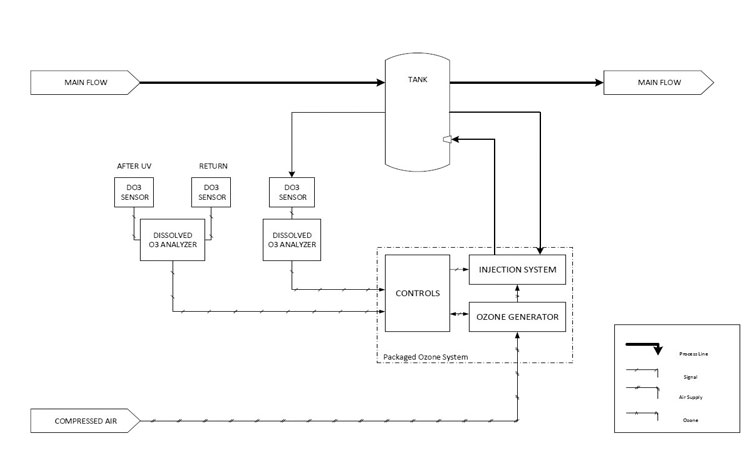
Purifying potable water sufficiently for use in the pharmaceutical industry usually requires a series of purification stages. A 3 the challenge the validation and qualification of water purification systems are a fundamental part of good automated manufacturing practice gamp and form an integral part of the gamp inspection. Distillation reverse osmosis de ionization filtration or equivalent means. Though usp defines the water quality standards it does not specify the methods for treating water to meet usp standards.