Purified Water System In Pharmaceutical Industry Ppt

Fda letter to the pharmaceutical industry re.
Purified water system in pharmaceutical industry ppt. Purified water system in pharmaceuticals 1. Michels bureau of drugs aug. Within the pharmaceutical industry water is most commonly used in liquid form not only as an ingredient in many formulations but also as a cleaning agent. Design validation of water systems.
Obtained by a suitable process usp. It is necessary to analyze the purified water and water for injection wfi for microbial contamination. Therefore this water treatment technology focuses on altering the ionic composition in a desirable direction. Due to its.
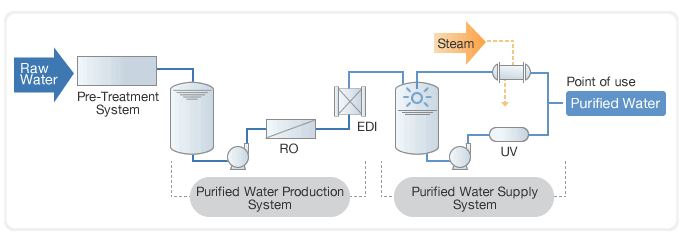
Microbial contamination in water may cause contamination in pharmaceutical products. Pharmaceutical water system ppt pharmaceutical water systems purified water specification as per usp ppt pdf pharmaceutical water system validation identification of microorganisms. Fda inspection technical guide number 36 reverse. Ion exchange reverse osmosis ro and distillation.
Design of the system. Sampling preservation and storage procedure of water sample. Operational considerations 83 7 1 start up and commissioning of water systems 83 7 2 qualification 83 7 3 continuous. Water is used as ingredient and solvent in the processing formulation and manufacture of pharmaceutical products active pharmaceutical ingredients apis and intermediates compendial articles and analytical reagents.
Pharmaceutical water system ppt what is pharmaceutical water. During this period sampling shall be done as per the routine sampling plan which will include testing of samples from each. Phase iii validation study. This general information chapter provides additional information about water its quality attributes that are not included within a water monograph processing techniques that can be used to improve water.
Ion exchange is one of the most desirable types of purified water treatments. Production of purified water highly purified water pyrogen free water and wfi to international pharmaceutical standards is widely recognised as a critical process. The third phase of validation is designed to demonstrate that when the water distribution system is operated in accordance with the sops over a long period of time one year it should consistently produce water of desired quality attributes. It involves separation of water soluble undesirable ions and exchanging those ions with desirable ones.
Validation and control of deionized water systems daniel l. 6 2 materials that come into contact with systems for water for pharmaceutical use 78 6 3 system sanitization and bioburden control 80 6 4 storage vessel requirements 80 6 5 requirements for water distribution pipework 81 7. Water is used in various processes in pharmaceutical manufacturing. Obtained by a suitable process conductivityconductivity 1 3 µs cm 25º c 1 3 µs cm 25º c total organic carbon toc 500 ppbtotal organic carbon toc 500 ppb microbial 100 cfu mlmicrobial 100 cfu ml.